Candida albicans bloodstream infection is increasingly frequent and can result in disseminated candidiasis associated with high mortality rates. To analyze the innate immune response against C. albicans, fungal cells were added to human whole-blood samples. After inoculation, C. albicans started to filament and predominantly associate with neutrophils, whereas only a minority of fungal cells became attached to monocytes. While many parameters of host-pathogen interaction were accessible to direct experimental quantification in the whole-blood infection assay, others were not. To overcome these limitations, we generated a virtual infection model that allowed detailed and quantitative predictions on the dynamics of host-pathogen interaction. Experimental time-resolved data were simulated using a state-based modeling approach combined with the Monte Carlo method of simulated annealing to obtain quantitative predictions on a priori unknown transition rates and to identify the main axis of antifungal immunity. Results clearly demonstrated a predominant role of neutrophils, mediated by phagocytosis and intracellular killing as well as the release of antifungal effector molecules upon activation, resulting in extracellular fungicidal activity. Both mechanisms together account for almost of C. albicans killing, clearly proving that beside being present in larger numbers than other leukocytes, neutrophils functionally dominate the immune response against C. albicans in human blood. A fraction of C. albicans cells escaped phagocytosis and remained extracellular and viable for up to four hours. This immune escape was independent of filamentation and fungal activity and not linked to exhaustion or inactivation of innate immune cells. The occurrence of C. albicans cells being resistant against phagocytosis may account for the high proportion of dissemination in C. albicans bloodstream infection. Taken together, iterative experiment–model–experiment cycles allowed quantitative analyses of the interplay between host and pathogen in a complex environment like human blood.
ODE/SBM Parameter Estimator
The ODE/SBM parameter estimator is a framework for simulating, estimating, and screening ordinary differential equations (ODEs) or state-based models (SBMs)…
Peroxisome motility analysis
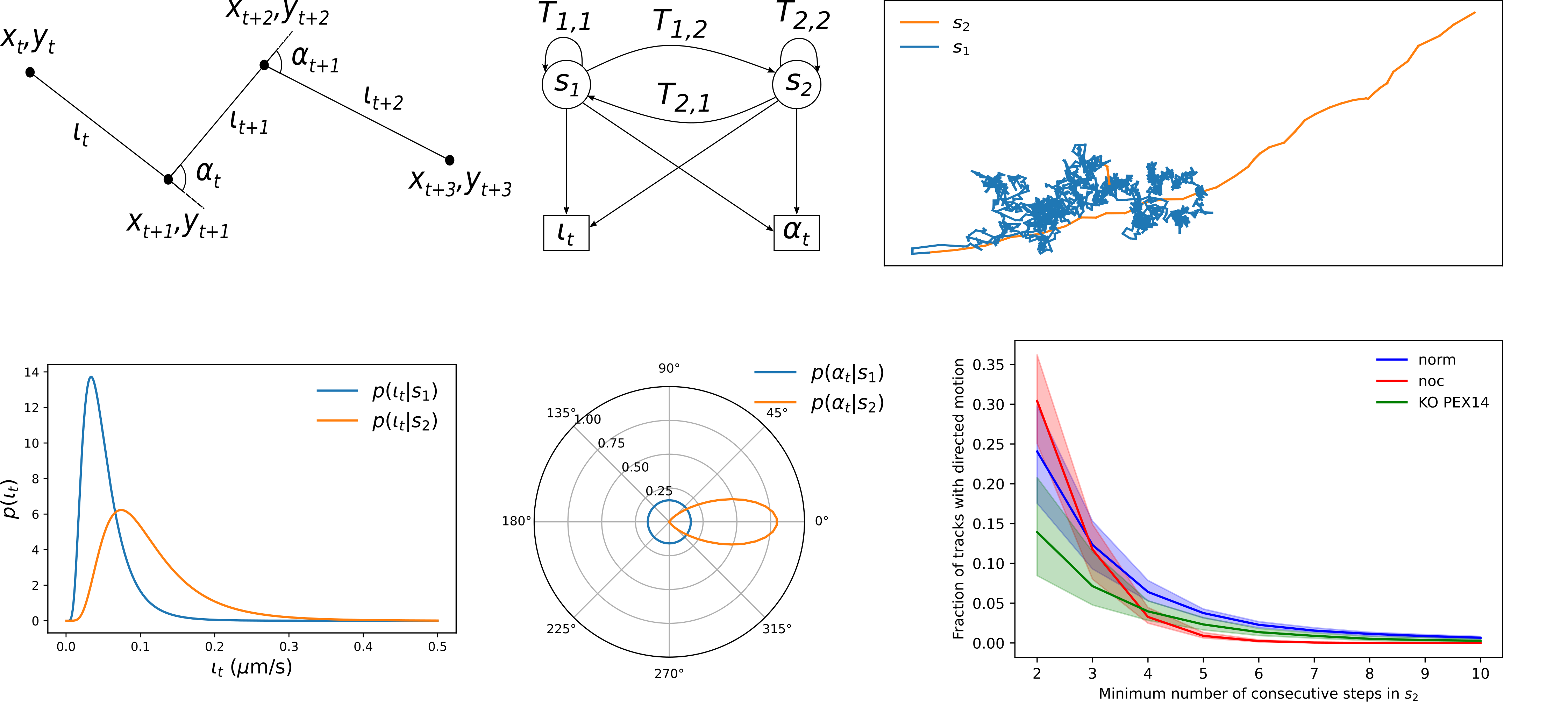
Peroxisome motility analysis using a Hidden Markov Model (HMM) enables the automatic identification and quantification of migration segments, thereby enhancing…