The ability of pathogens to evade phagosomal killing is critical for their pathogenicity. Previously, we had identified the HscA effector protein in the clinically important fungal pathogen Aspergillus fumigatus, which redirects conidia-containing phagosomes from the degradative to the non-degradative pathway. Here, we discovered a pathogenic form of this surface protein, determined by a single tyrosine residue (Y) at position 596, which is lacking in most of fungi analyzed, that have a leucine (L) instead. Y596 enables HscA to penetrate the phagosomal membrane. In line, the introduction of a single L-to-Y exchange in the orthologous Ssb protein of Saccharomyces cerevisiae enabled the protein to penetrate phagosomal membranes that was reduced by deletion of one of the two Y-encoding SSB genes in the pathogenic fungus Candida glabrata. These data suggest a convergent evolution of HscA/Ssb proteins among human-pathogenic fungi and that a single amino acid exchange determines a virulence factor.
Confrontation assay model
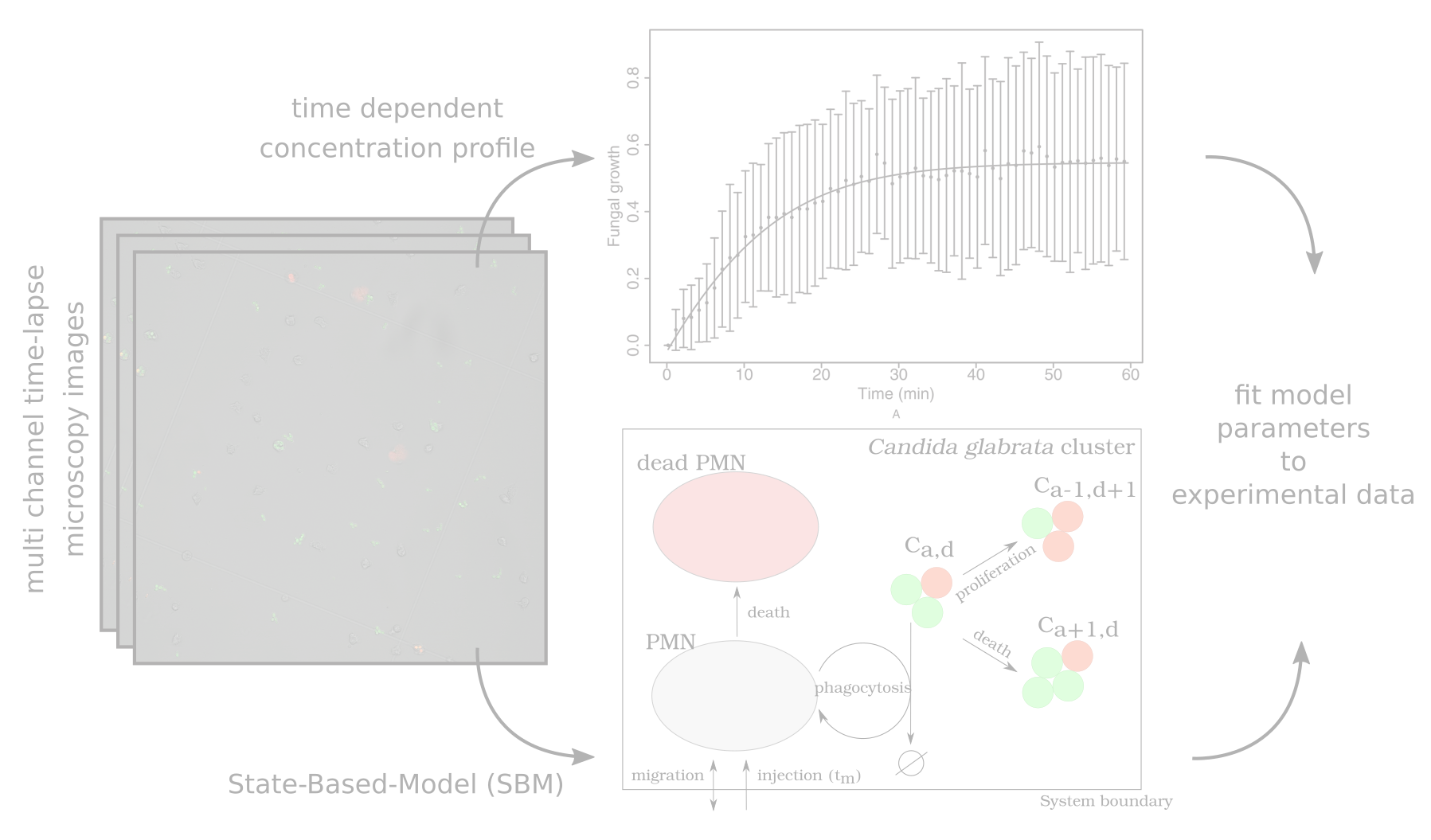
In this project a virtual confrontation assay of polymorphonuclear neutrophils (PMN) and Candida glabrata cell cluster is modelled. Bases on…
DeconvTest
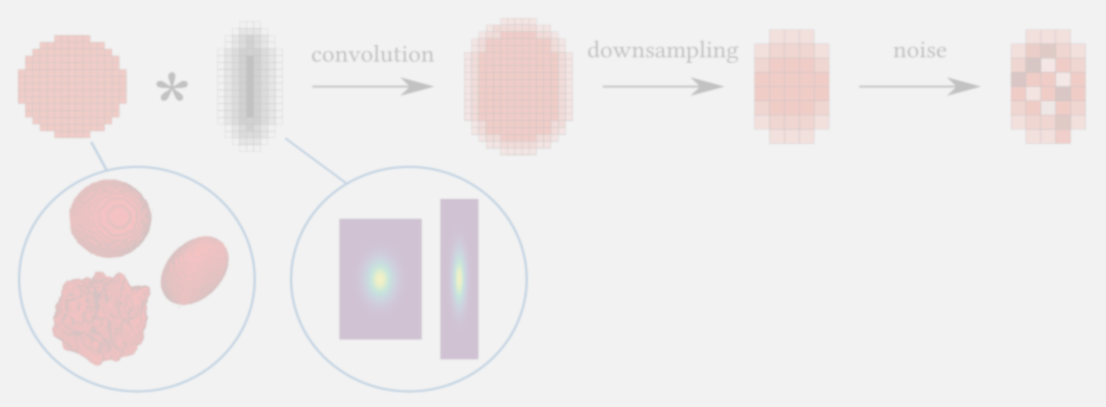
For many existing deconvolution methods, it is challenging to choose the method and its parameters most appropriate for particular image…