The ability of pathogens to evade phagosomal killing is critical for their pathogenicity. Previously, we had identified the HscA effector protein in the clinically important fungal pathogen Aspergillus fumigatus, which redirects conidia-containing phagosomes from the degradative to the non-degradative pathway. Here, we discovered a pathogenic form of this surface protein, determined by a single tyrosine residue (Y) at position 596, which is lacking in most of fungi analyzed, that have a leucine (L) instead. Y596 enables HscA to penetrate the phagosomal membrane. In line, the introduction of a single L-to-Y exchange in the orthologous Ssb protein of Saccharomyces cerevisiae enabled the protein to penetrate phagosomal membranes that was reduced by deletion of one of the two Y-encoding SSB genes in the pathogenic fungus Candida glabrata. These data suggest a convergent evolution of HscA/Ssb proteins among human-pathogenic fungi and that a single amino acid exchange determines a virulence factor.
KITE – EPIDEMIOLOGICAL MODELLING OF INFECTION SPREAD IN CHILD CARE FACILITIES
KITE (KIndergarten Test scenario Evaluator) is a state-based model for simulating the spread of different airborne viruses in kindergartens. The…
Mcat
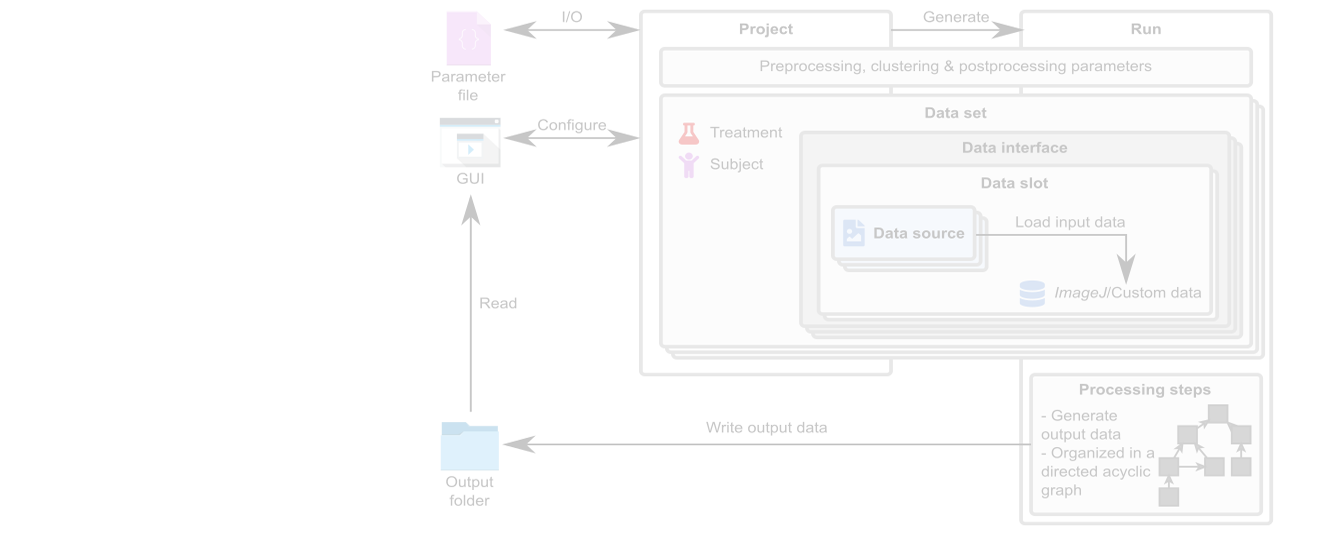
Our MSOT cluster analysis toolkit enables the quantitative and automated analysis of multispectral optoacoustic tomography (MSOT) images. The pharmacokinetics of…