Extracellular vesicles (EVs) have gained attention as facilitators of intercellular as well as interkingdom communication during host-microbe interactions. Recently we showed that upon infection, host polymorphonuclear leukocytes produce antifungal EVs targeting the clinically important fungal pathogen Aspergillus fumigatus; however, the small size of EVs (< 1 µm) complicates their functional analysis. Here, we employed a more tractable, reporter-based system to label host alveolar epithelial cell-derived EVs and enabled their visualisation during in vitro A. fumigatus interaction. Fusion of EV marker proteins (CD63, CD9, and CD81) with a Nanoluciferase (NLuc) and a green fluorescent protein (GFP) facilitated their relative quantification by luminescence and visualization by a fluorescence signal. The use of an NLuc fused with a GFP is advantageous as it allows for quantification and visualisation of EVs simultaneously without additional external manipulation and to distinguish subpopulations of EVs. Using this system, visualisation and tracking of EVs was possible using confocal laser scanning microscopy and advanced imaging analysis. These experiments revealed the propensity of host cell-derived EVs to associate with the fungal cell wall and ultimately colocalize with the cell membrane of A. fumigatus hyphae in large numbers. In conclusion, we have created a series of tools to better define the complex interplay of host-derived EVs with microbial pathogens.
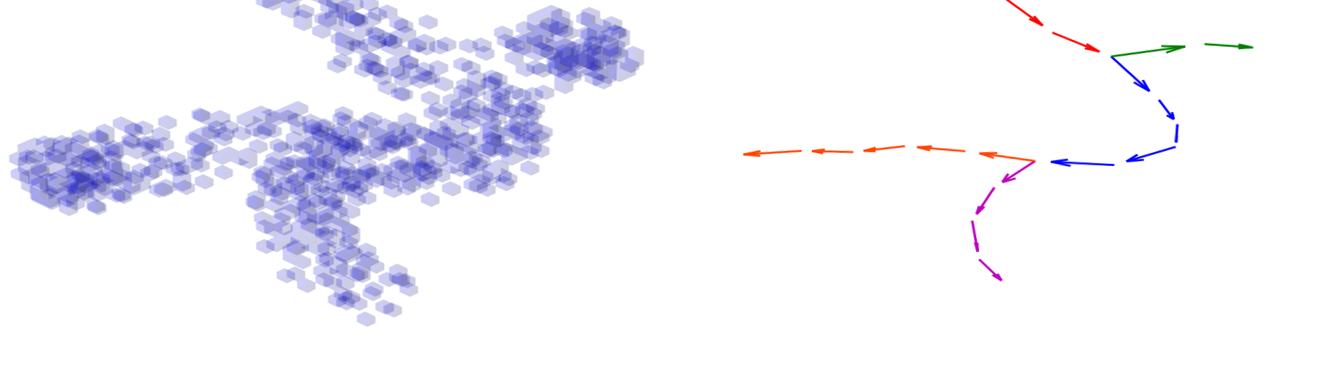
3D-HyTracer
3D-HyTracer is an image analysis tool for automated tracing of fungal hyphae. It creates a low-memory and easily accessible spatial…
ABM
The agent-based modelling framework is a generalized framework that is able to capture spatio-temporal dynamics of processes in host-pathogen interactions…